Project 03
The role of platelet GPVI signalling shutdown in cardiogenic shock and the transition to sepsis
Project details
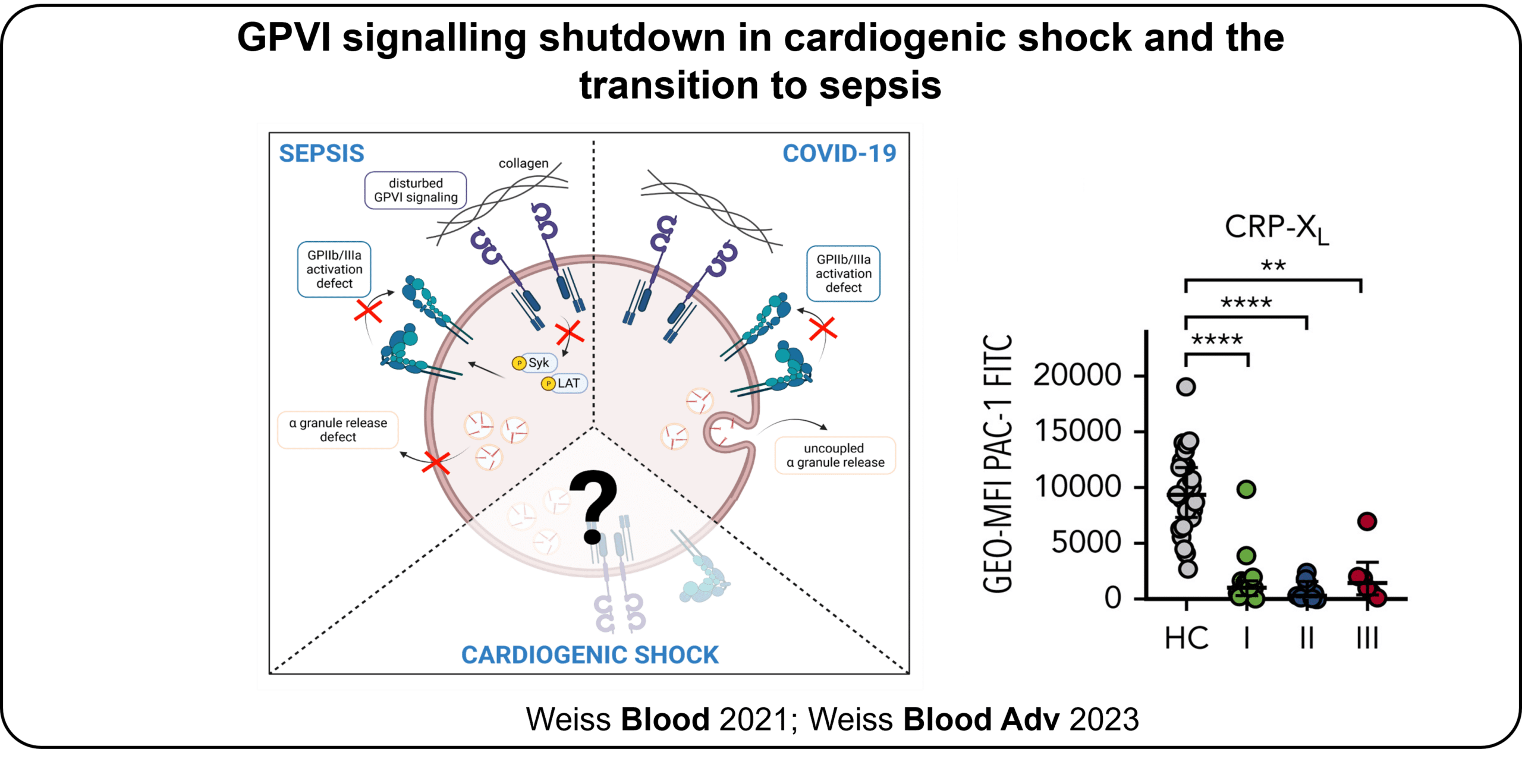
Sepsis is of paramount clinical relevance with high mortality rates and an increased risk of bleedings. Interestingly, patients with cardiogenic shock (CS) of different causes have an increased risk of developing sepsis. We identified that platelet glycoprotein (GP)VI responsiveness to agonists (CRP-XL, convulxin) is blunted in patients with sepsis. The potential changes in platelet GPVI function in CS and during sepsis onset remain however unknown.
This projects aims to identify the molecular mechanisms leading to blunted platelet function via GPVI signalling shutdown in sepsis and provide evidence if patients with cardiogenic shock have the same GPVI signalling defect as in sepsis/septic shock and does this affect the transition to sepsis. A particular question is how the defective GPVI signalling can be transferred to platelets of healthy donors, when incubated in whole blood of sepsis patients, suggesting a yet unknown cellular component in sepsis, independent of the underlying pathogen. Furthermore, the project aims to decipher the changes in function of platelet GPVI signalling in cardiogenic shock. The ultimate goal is to identify new therapeutic targets (VI) to successfully modify platelet GPVI signalling shutdown and thus thrombo-inflammatory conditions in sepsis and cardiogenic shock. P03 will use state-of-the-art methods including coagulation flow chambers and confocal immunofluorescence microscopy.
ECR.P03.04 Changes and systemic modulation of the GPVI signalling cascade in cardiogenic shock (CS) and sepsis: molecules and pathways – will (i) study platelet function and develop new methods in patients with CS, especially in the transition to sepsis; (ii) use biochemical and cell biological approaches to identify which triggers lead to the shutdown of platelet GPVI, will (iii) study the altered GPVI signalling cascade in CS and sepsis using systems biological approaches including platelet and neutrophil (phospho-)proteomics, neutrophil scRNA sequencing to decipher the underlying signalling pathways; (iv) study how thrombus formation occurs under defined shear rates using a coagulation flow chamber model with staining for platelets, neutrophils and markers of the coagulation system, and(v) adopt a mouse model for sepsis to further investigate the signalling events in genetically modified mice. .
References
- Weiss LJ, Manukjan G, Pflug A, Winter N, Weigel M, Nagler N, Kredel M, Lam TT, Nieswandt B, Weismann D, and Schulze H (2021). Acquired platelet GPVI receptor dysfunction in critically ill patients with sepsis. Blood 137, 3105-3115. doi: 10.1182/blood.2020009774
- Weiss LJ, Drayss M, Mott K, Droste M, Just B, Arold AM, Nieswandt B, Weismann D, Stegner D, and Schulze H (2022). The homophilic CD84 receptor is upregulated on platelets in COVID-19 and sepsis. Thromb Res 220, 121-124. doi: 10.1016/j.thromres.2022.10.011
- Weiss LJ, Drayss M, Manukjan G, Zeitlhofler M, Kleiss J, Weigel M, Herrmann J, Mott K, Beck S, Burkard P, Lam TT, Althaus K, Bakchoul T, Frantz S, Meybohm P, Nieswandt B, Weismann, D, and Schulze H (2023). Uncoupling of platelet granule release and integrin activation suggests GPIIb/IIIa as a therapeutic target in COVID-19. Blood Adv 7, 2324-2338. doi: 10.1182/bloodadvances.2022008666
- Herrmann J*, Weiss LJ*, Just B, Mott K, Drayss M, Kleiss J, Riesner J, Notz Q, Röder D, Leyh R, Beck S, Weismann D, Nieswandt B, Lotz C, Meybohm P§, Schulze H§ (2024). ECMO aggravates platelet GPV shedding and δ-granule deficiency in COVID-19-associated acute respiratory distress syndrome. JTH 2024. May 17:S1538-7836(24)00290-3. doi: 10.1016/j.jtha.2024.05.008. (* shared first-authors, § shared last-authors)
Desirable student skills
- Experience with cell culture, molecular biology / biochemical techniques (qPCR, Western blot, genotyping), immunocytochemistry and flow cytometry. Previous work with experimental animals would be advantageous.
- Experience with state-of-the-art immunofluorescence imaging methods. Profound knowledge in cell biology as well as previous work with cell culture would be desired.
Supervisory team

Prof. Dr. Harald Schulze
Professor (W2) Experimental Haemostaseology

