Stroke is among the leading causes for death and disability worldwide. According to the world stroke organization, there are over 12.2 million new strokes each year and 6.5 million people die from stroke annually. The majority of strokes are ischemic, meaning an occlusion of a brain supplying vessel leading to ischemia of the distal brain areas. On the other side, approximately a quarter of all stroke cases are caused by intracranial hemorrhages.
In the case of an acute ischemic stroke, therapeutic options are limited to either chemical thrombolysis or mechanical thrombectomy trying to restore blood supply as fast as possible. However, successful removal of the initial thrombus does not guarantee sufficient reperfusion and the infarct might grow further. Our group is investigating the reasons why reperfusion fails and aims to identify new therapeutic targets preventing secondary infarct growth. We are working on revealing the cellular interactions underlying the thrombo-inflammatory state of the ischemic and postischemic brain that drive disease progression following the initial insult.
Intracerebral hemorrhage (ICH) can converse from ischemic stroke or develop acutely mostly in patients with elevated blood pressure or associated with anti-coagulant therapy. In models of ICH, we study the underlying pathomechanisms and aim to elucidate whether targeting platelets can promote hemostasis to improve the outcome.
Upon a large brain vessel occlusion, the area surrounding the immediately evolving infarct (penumbra) is still provisionally nourished by collateral blood flow from the brain surface. However, this is insufficient to prevent tissue death in the long term. The collapse of the macro-and microvascular collateral flow (collateral failure) leads to penumbral tissue death and infarct progression. Mounting preclinical and clinical evidence indicates that platelet- and leukocyte-driven thrombo-inflammation is a pivotal modulator. Therefore, we are investigating the contribution of platelet receptors and their proximal signaling in pathological intravascular platelet-immune cell interactions which induce vascular dysfunction, collateral failure, and damage of the blood brain barrier (BBB) leading to the detrimental remote effects on the brain parenchyma.
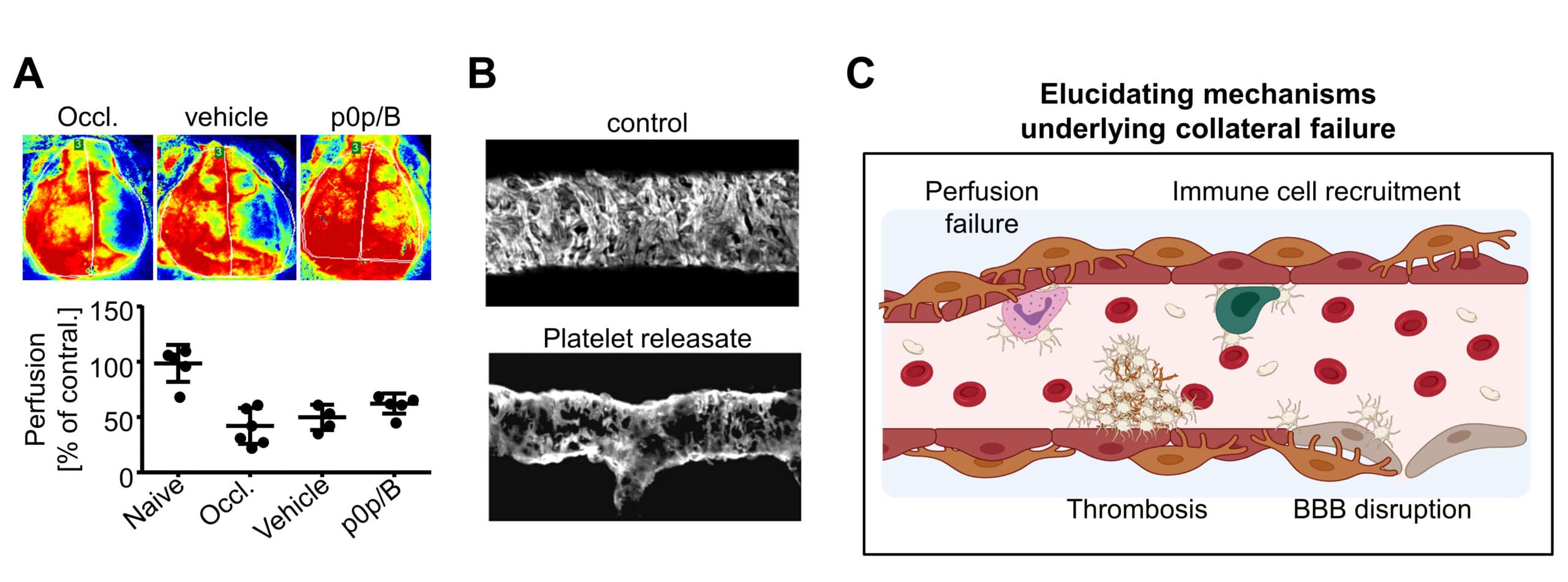
Contribution of platelets to the failure of the cerebral microcirculation upon ischemic stroke. (A) Laser doppler perfusion monitoring of the cerebral blood flow (CBF) in acute ischemic stroke. Blocking platelet adhesion (p0p/B) improves CBF in the ipsilateral hemisphere. Red: maximum perfusion, blue: no perfusion. (B) Pericyte sheath surrounding blood vessels is altered when being exposed to platelet granule content (platelet releasate). Example from the cremaster muscle – images were kindly provided by the Girbl-Lab. (C) Schematic overview of proposed processes being involved in driving thrombo-inflammation in the brain in acute ischemic stroke. Platelets and immune cells act on the cerebral vasculature causing perfusion failure and disruption of the blood brain barrier (BBB) worsening tissue damage. This cartoon was created using BioRender.
To further investigate the collapse of cerebral microcirculation, reduction in cerebral blood flow and BBB breakdown, we are interested in the interaction of platelets with endothelial cells or pericytes. Pericytes cover endothelial cells and are important mediators and regulators of blood flow, barrier integrity and immune cell trafficking and inflammation. There is evidence that platelets are engaged in direct contacts and interactions with pericytes, and some components of platelet granule secretion facilitate platelet-pericyte or platelet-endothelial interactions in a paracrine fashion as well.
Using advanced fluorescence microscopy like light sheet fluorescence microscopy (LSFM) and intravital 2-photon microscopy (2P-IVM) combined with respective in vitro models and a large toolbox of antibodies and genetic models, we aim to visualize the interplay of platelets with immune cells, endothelial cells or pericytes at the vascular interface during large vessel occlusion and thrombo-inflammation. The ultimate goal of this is to identify new therapeutic targets for treating ischemic stroke.
Key publications:
Göb V, Voll MG, Zimmermann L, Hemmen K, Stoll G, Nieswandt B, Schuhmann MK, Heinze KG, Stegner D. Infarct growth precedes cerebral thrombosis following experimental stroke in mice. Sci Rep. 2021; 11(1):22887. doi: 10.1038/s41598-021-02360-6.
Schuhmann MK, Stoll G, Bieber M, Vögtle T, Hofmann S, Klaus V, Kraft P, Seyhan M, Kollikowski AM, Papp L, Heuschmann PU, Pham M, Nieswandt B, Stegner D. CD84 Links T Cell and Platelet Activity in Cerebral Thrombo-Inflammation in Acute Stroke. Circ Res. 2020; 127(8):1023-1035. doi: 10.1161/CIRCRESAHA.120.316655.
Stegner D, Hofmann S, Schuhmann MK, Kraft P, Herrmann AM, Popp S, Höhn M, Popp M, Klaus V, Post A, Kleinschnitz C, Braun A, Meuth SG, Lesch KP, Stoll G, Kraft R, Nieswandt B. Loss of Orai2-Mediated Capacitative Ca(2+) Entry Is Neuroprotective in Acute Ischemic Stroke. Stroke. 2019; 50(11):3238-3245. doi: 10.1161/STROKEAHA.119.025357.
Schuhmann MK, Bieber M, Franke M, Kollikowski AM, Stegner D, Heinze KG, Nieswandt B, Pham M, Stoll G. Platelets and lymphocytes drive progressive penumbral tissue loss during middle cerebral artery occlusion in mice. J Neuroinflammation. 2021; 18(1):46. doi: 10.1186/s12974-021-02095-1.
The majority of strokes are caused by an occlusion of a brain supplying vessel leading to ischemia of the distal brain areas. Consequently, anti-platelet therapies are widely used in stroke prevention (to reduce the risk of a second ischemic event). However, in the context of acute ischemic stroke anti-platelet therapies are avoided due to the risk of peripheral bleeding as well as hemorrhagic transformation within the brain. Instead, the therapeutic options are limited to recanalization interventions (thrombolysis and thrombectomy), which benefit only a fraction of the patients. Thus, additional adjunctive therapies applicable to acute ischemic stroke are needed.
Platelets play a significant role in secondary infarct growth following ischemic stroke. Experimental and clinical data suggest that secondary infarction is not caused by platelet aggregation-related thrombosis within the microcirculation but should be attributed to platelets’ thrombo-inflammatory activity, like the recruitment of immune cells. This offers the intriguing possibility to target platelets’ inflammatory activity without increasing the risk of intracranial hemorrhages.
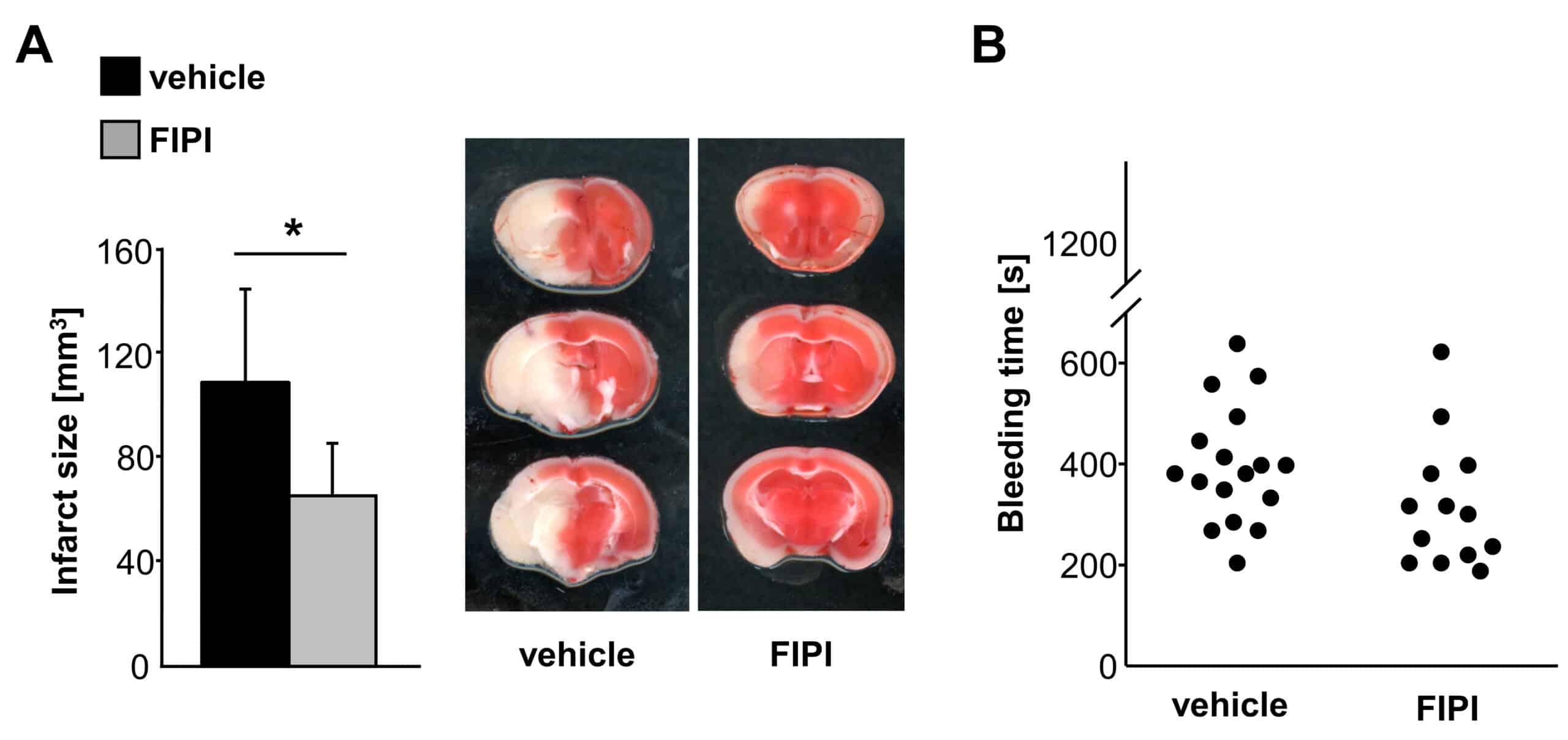
The treatment with 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a selective inhibitor of phospholipase D (PLD, an enzyme that contributes to the signaling cascade downstream of GPIbα) exerts protection from ischemic stroke without impairing hemostasis. (A) Brain infarct volumes of vehicle and FIPI-treated mice and representative images of 3 corresponding coronal sections subjected to the transient middle cerebral artery occlusion (tMCAO), which were stained with TTC. (B) Tail bleeding times of vehicle- and FIPI-treated mice. These images are taken from Stegner et al., ATVB 2013.
We assess the potential of experimental targeting strategies with the help of in-house generated antibodies, small molecules (obtained in collaboration with partners from the pharmaceutical industry) and genetic models (e.g. humanized mice).
Key publications:
Brown HC, Beck S, Navarro S, Di Y, Soriano Jerez EM, Kaczmarzyk J, Thomas SG, Mirakaj V, Watson SP, Nieswandt B, Stegner D. Antibody-mediated depletion of human CLEC-2 in a novel humanised mouse model. Blood Adv. 2022; 7(6):997-1000. doi: 10.1182/bloodadvances.2021006463.
van Eeuwijk JMM*, Stegner D*, Lamb DJ, Kraft P, Beck S, Thielmann I, Kiefer F, Walzog B, Stoll G, Nieswandt B. The novel oral Syk inhibitor, Bl1002494, protects mice from arterial thrombosis and thrombo-inflammatory brain infarction. Arterioscler Thromb Vasc Biol. 2016; 36(6):1247-53. doi: 10.1161/ATVBAHA.115.306883.
Stegner D*, Thielmann I*, Kraft P, Frohman MA, Stoll G, Nieswandt B. Pharmacological inhibition of phospholipase D protects mice from occlusive thrombus formation and ischemic stroke. Arterioscler Thromb Vasc Biol. 2013; 33(9):2212-7. doi: 10.1161/ATVBAHA.113.302030.
Intracranial hemorrhage (ICH, bleeding in the brain) is a devastating condition with limited treatment options. It can occur due to various causes, like the rupture of a cerebral vessel due to hypertension (high blood pressure), cerebral amyloid angiopathy (the accumulation of amyloid protein in the walls of blood vessels) or vascular anomalies. Additionally, traumatic brain injury or hemorrhagic transformation following ischemic stroke can result in intracranial hemorrhage. Treatment may involve addressing the specific cause, e.g. controlling blood pressure, and, in some cases, surgical intervention to stop bleeding or repair vascular abnormalities. The prognosis varies depending on the severity of the hemorrhage and the underlying condition. Pro-coagulant therapy may offer potential benefits in controlling bleeding in intracranial hemorrhage, but its use is associated with significant risks, particularly for thromboembolic events. Thus, ongoing research is crucial to further clarify the role and safety of pro-coagulant therapy in the context of intracranial hemorrhage. In this regard, blocking the generation of soluble platelet glycoprotein V (GPV), which dampens fibrin formation and therefore limits hemostatic activity, could be an attractive strategy, as soluble GPV is only formed at sites of coagulation and a localized pro-coagulant activity should have a better safety profile. Using antibodies generated by the Würzburg platelet groups against GPV and other platelet receptors in combination with experimental ICH models we want to assess novel therapeutic strategies to dampen ICH.
Key publications:
Beck S, Öftering P, Li R, Hemmen K, Nagy M, Wang Y, Zarpellon A, Schuhmann MK, Stoll G, Ruggeri ZM, Heinze KG, Heemskerk JWM, Ruf W*, Stegner D*, Nieswandt B*. Platelet glycoprotein V spatio-temporally controls fibrin formation. Nat Cardiovasc Res. 2023;2(4):368-382. doi: 10.1038/s44161-023-00254-6.
Navarro S, Stegner D, Nieswandt B, Heemskerk JWM, Kuijpers MJE. Temporal Roles of Platelet and Coagulation Pathways in Collagen- and Tissue Factor-Induced Thrombus Formation. Int J Mol Sci. 2021; 23(1):358. doi: 10.3390/ijms23010358.
Deppermann C, Kraft P, Volz J, Schuhmann MK, Beck S, Wolf K, Stegner D, Stoll G, Nieswandt B. Platelet secretion is crucial to prevent bleeding in the ischemic brain but not in the inflamed skin or lung in mice. Blood. 2017; 129(12):1702-1706. doi: 10.1182/blood-2016-12-750711.
Cerebral venous (sinus) thrombosis, CV(S)T is a rare type of stroke of mainly idiopathic onset. While a significant contribution of platelets is known and widely accepted in arterial thrombosis, this was less clear in venous thrombosis, but is increasingly being considered. So far, anticoagulation is the mainstay of acute and subacute treatment for CVT. We have recently shown that fab-fragments of the anti-CLEC2 (an activatory hemITAM receptor on the platelet surface) antibody, INU1, induce pathological platelet activation in vivo, leading to foudroyant CVT accompanied by severe neurological symptoms. Using this as a model, we demonstrated that cooperative signaling between the two platelet receptors CLEC2 and GPIIb/IIIa is an important trigger of CVT, opening new treatment avenues.
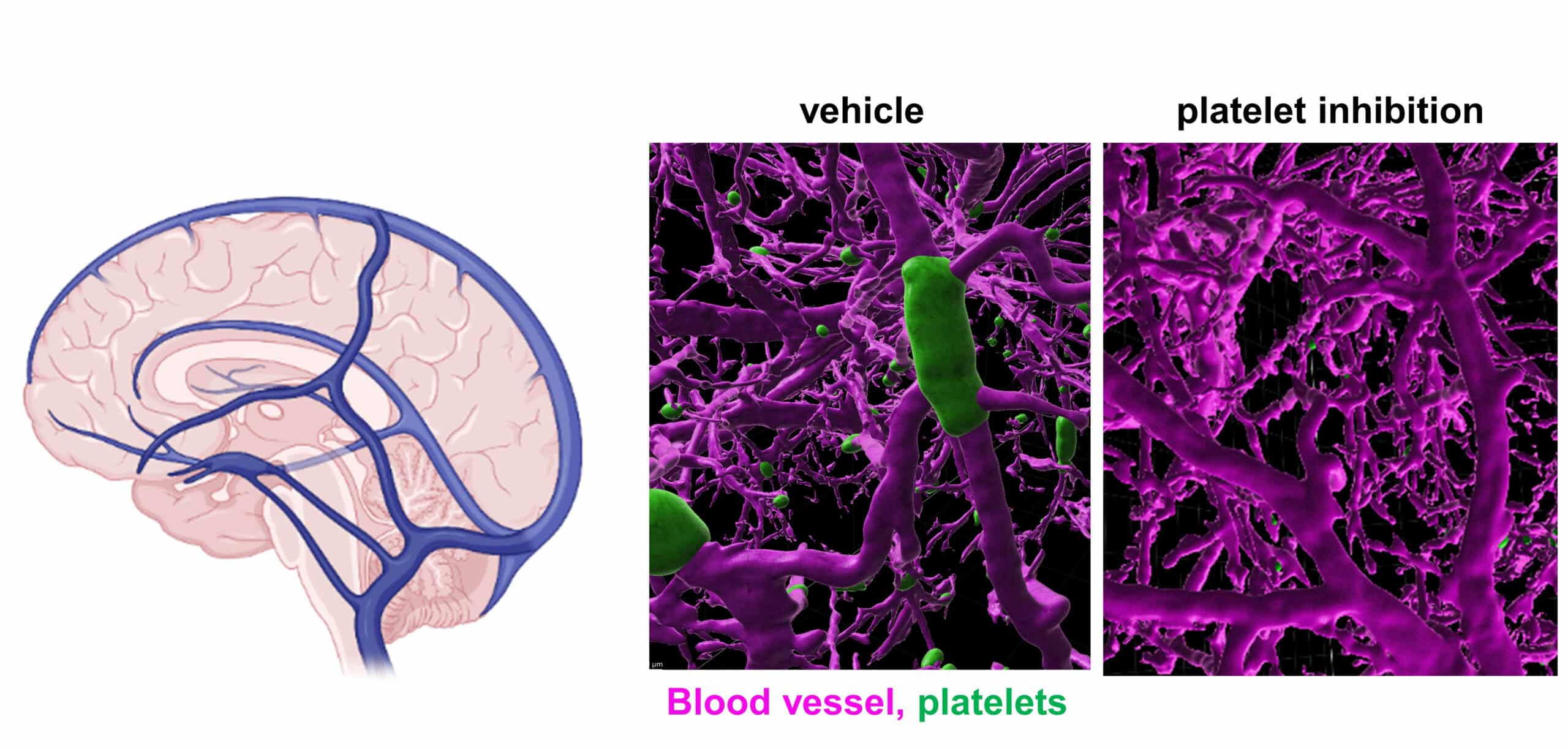
Cerebral venous thrombosis. Schematic overview of the major venous system of the brain. 3D reconstruction from light sheet fluorescence microscopy from the murine brain vasculature (magenta) following INU1-fab induced cerebral venous thrombosis (left). Thrombi are visible in green. Platelet inhibition prevents venous thrombus formation (right).
Key publications:
Stegner D, Göb V, Krenzlin V, Beck S, Hemmen K, Schuhmann MK, Schörg BF, Hackenbroch C, May F, Burkard P, Pinnecker J, Zernecke A, Rosenberger P, Greinacher A, Pichler BJ, Heinze KG, Stoll G, Nieswandt B. Foudroyant cerebral venous (sinus) thrombosis triggered through CLEC-2 and GPIIb/IIIa dependent platelet activation. Nat Cardiovasc Res. 2022; 1:132-141. doi: 10.1038/s44161-021-00017-1
Göb V, Voll MG, Zimmermann L, Hemmen K, Stoll G, Nieswandt B, Schuhmann MK, Heinze KG, Stegner D. Infarct growth precedes cerebral thrombosis following experimental stroke in mice. Sci Rep. 2021; 11(1):22887. doi: 10.1038/s41598-021-02360-6.
Maqsood Z, Clark JC, Martin EM, Cheung YFH, Morán LA, Watson SET, Pike JA, Di Y, Poulter NS, Slater A, Lange BMH, Nieswandt B, Eble JA, Tomlinson MG, Owen DM, Stegner D, Bridge LJ, Wierling C, Watson SP. Experimental validation of computerised models of clustering of platelet glycoprotein receptors that signal via tandem SH2 domain proteins. PLoS Comput Biol. 2022; 18:e1010708. doi: 10.1371/journal.pcbi.1010708.