The liver is the largest glandular organ in the body and known for its role in metabolism, digestion, and detoxification. But with the majority of the hepatic blood supply coming directly from the gut via the portal vein, the liver also functions as an important first barrier for microbial and environmental antigens, linking it integrally to the body’s immune system. This is reflected in the unique architecture of the liver. The hepatic sinusoids are lined by sinusoidal endothelium cells (LSECs) that are separated from the hepatocytes by the space of Disse. Hepatocytes are highly metabolically active and produce most blood coagulation factors, while LSECs clear waste products and small immune complexes through endocytic receptors. They are fenestrated to allow for easy interaction of liver resident macrophages, the Kupffer cells (KCs), residing in the space of Disse, with blood flowing from the portal to the central vein. Constantly changing metabolic activity and tissue remodeling, combined with regular exposure to antigens, results in a delicate homeostasis of inflammation and resolution within the liver that needs a tight regulation to prevent chronic inflammation.
We study the role of platelets as regulatory and effector cells within this unique environment. State-of-the-art intravital confocal microscopy – in combination with a large toolbox of antibodies and genetic models to visualize cells of interest – allows us to track interactions of individual platelets with immune cells and the liver endothelium in real time across different conditions, ranging from the clearance of platelets to hepatic ischemia reperfusion injury, sterile inflammation, and sepsis.
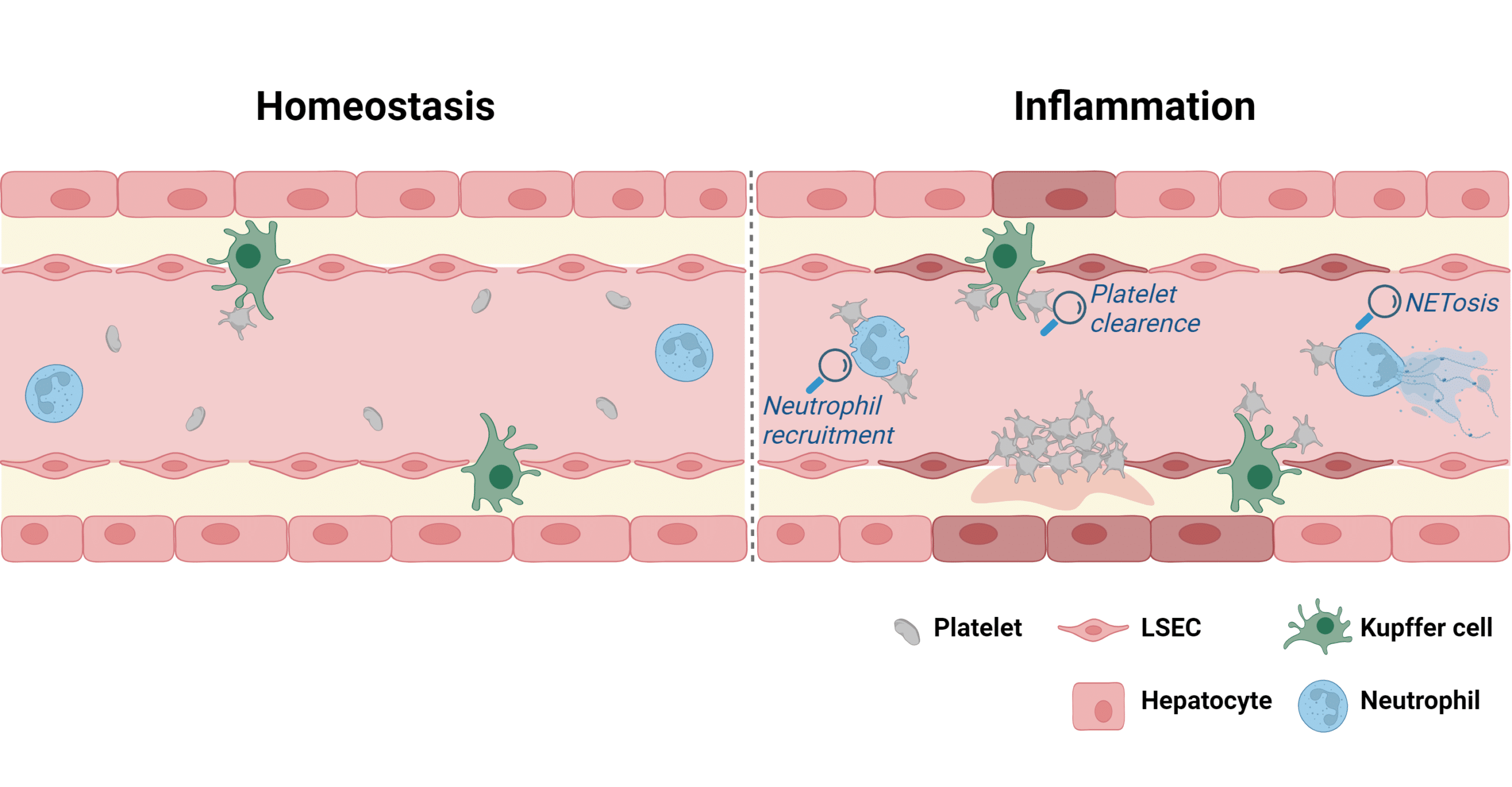
Liver inflammation. The hepatic sinusoids are lined by LSECs that are separated from the hepatocytes by the space of Disse. Kupffer cells reside in the space of Disse and clear ageing platelets and various pathogens from the circulation. Under inflammatory conditions clearance is enhanced, as platelets directly interact with pathogens and immune cells. Platelets have the capacity to recruit immune cells, such as neutrophils and modulate reactive oxygen production and NET formation. Platelets also maintain the vascular integrity during inflammation. This cartoon was generated using BioRender.
Key publications:
Stegner D, Popp M, Lorenz V, Wax JK, Gessner JE, Nieswandt B. FcγRIIB on liver sinusoidal endothelial cells is essential for antibody-induced GPVI ectodomain shedding in mice. Blood. 2016; 128(6):862-5. doi: 10.1182/blood-2016-05-714378.
Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, Leone V, Peiseler M, Surewaard BGJ, Rath D, Ali A, Wolf MJ, Drescher H, Healy ME, Dauch D, Kroy D, Krenkel O, Kohlhepp M, Engleitner T, Olkus A, Sijmonsma T, Volz J, Deppermann C, Stegner D, Helbling P, Nombela-Arrieta C, Rafiei A, Hinterleitner M, Rall M, Baku F, Borst O, Wilson CL, Leslie J, O’Connor T, Weston CJ, Adams DH, Sheriff L, Teijeiro A, Prinz M, Bogeska R, Anstee N, Bongers MN, Notohamiprodjo M, Geisler T, Withers DJ, Ware J, Mann DA, Augustin HG, Vegiopoulos A, Milsom MD, Rose AJ, Lalor PF, Llovet JM, Pinyol R, Tacke F, Rad R, Matter M, Djouder N, Kubes P, Knolle PA, Unger K, Zender L, Nieswandt B, Gawaz M, Weber A, Heikenwalder M. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019; 25(4):641-655. doi: 10.1038/s41591-019-0379-5.
Deppermann C, Cherpokova D, Nurden P, Schulz JN, Thielmann I, Kraft P, Vögtle T, Kleinschnitz C, Dütting S, Krohne G, Eming SA, Nurden AT, Eckes B, Stoll G, Stegner D*, Nieswandt B*. Gray platelet syndrome and defective thrombo-inflammation in NBEAL2-deficient mice. J Clin Invest. 2013; 123(8):3331-42. doi: 10.1172/JCI69210.