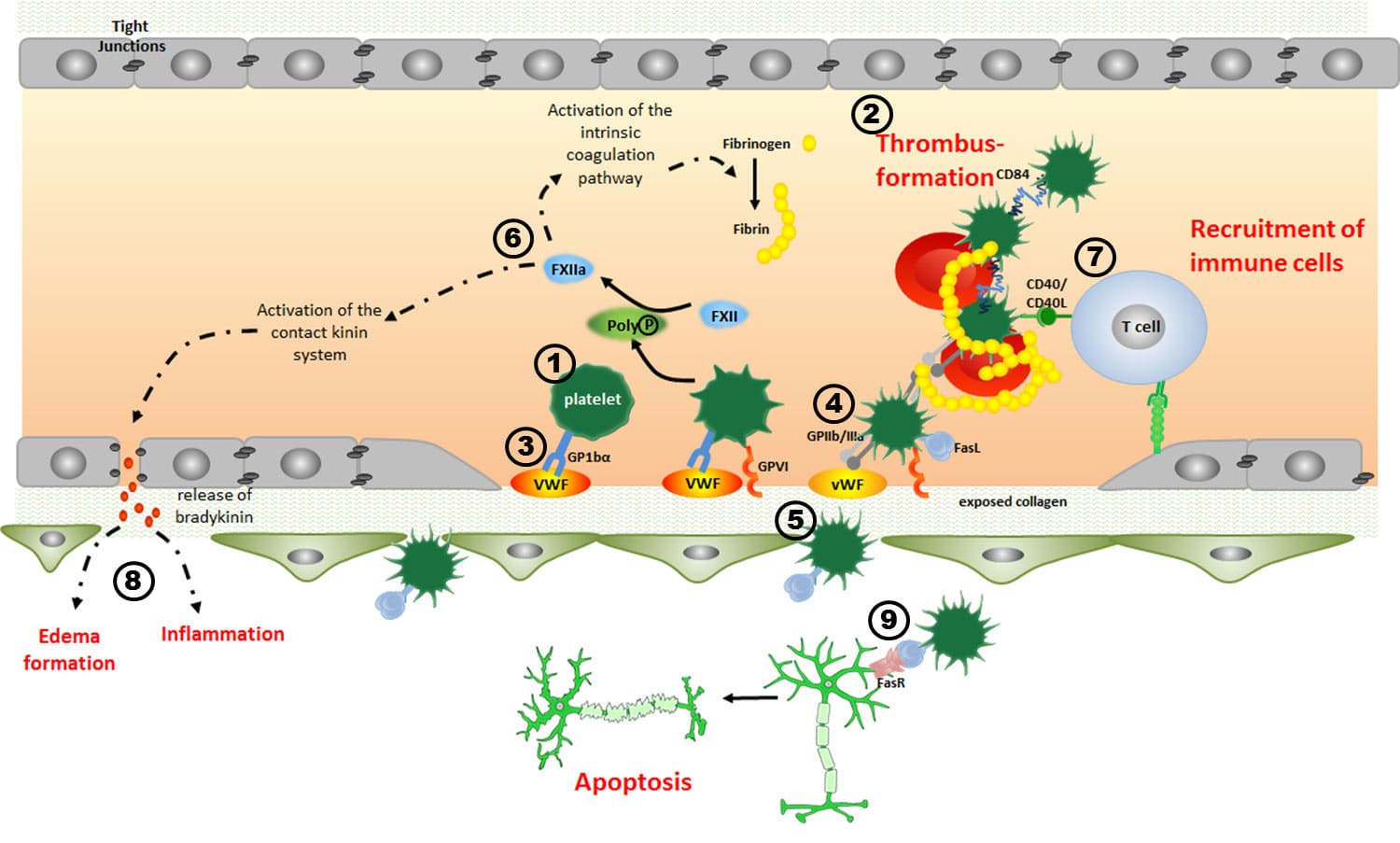
In recent years, our research groups have extensively characterized the pathophysiological role of platelets (1) as mediators of thrombus formation (2) during the acute phase of ischemic stroke. We were able to demonstrate, for example, that blocking platelet adhesion by interfering with the platelet glycoprotein (GP) receptor Ib (3) prevents microvascular thrombosis in the ischemic mouse brain without increasing the risk of intracranial haemorrhage. Surprisingly however, blocking of GPIIb/IIIa (4), the receptor of the final common pathway of platelet aggregation, did not protect from acute experimental stroke but caused a massive increase in intracranial bleedings (5). This indicates that apart from thrombosis other pathophysiological factors exist in stroke that drive secondary brain damage. Here, inflammation is one of the key candidates. Interestingly, in acute ischemic stroke, thrombus formation and inflammation are closely intertwined (“thrombo-inflammation”). For example, at the neurovascular interface platelets interact with endothelial cells (6) and T cells (7) and this interaction further promotes secondary tissue damage and capillary no-reflow (8). Since the majority of stroke patients is not eligible for early acute interventions such as recanalization strategies current therapeutic efforts aim to foster tissue remodelling and repair at more advanced stages. Here, the role of platelets is unknown. Recently, platelet activation in the CNS has emerged as an important factor in unexpected settings – e.g. in autoimmune diseases such as multiple sclerosis, where platelets accelerate organ damage via leukocyte recruitment and microglia activation. Platelets may also balance tissue homeostasis outside the vasculature, in ways not previously appreciated; e.g. they can induce apoptosis (9), an important process that has been shown to contribute to tissue remodelling in various settings. Supporting this, in models of acute ischemic stroke and retinal degeneration we have found that platelets are associated with non-leukocytic apoptotic cells.
The central hypothesis of this project is that platelets are central players of tissue remodelling and repair during the recovery phase (late phase) of an ischemic insult. We will analyse how the modification of platelet function and activation affects late-stage thrombosis, tissue inflammation and functional recovery. Moreover, we will characterize the role of platelets “outside” the vasculature – in particular, their potential to modulate apoptosis, angiogenesis and neuronal plasticity.